Research
The Detection Technology Group develops novel assay formats for diagnostics, environmental and food analysis, and drug discovery. The aim is to solve current problems related to industrial use with entirely new reagent-based analytical detection techniques. The technologies include label chemistry, lanthanide chelates, time-resolved luminescence, nanoparticle techniques and various specific and nonspecific assay and detection modes such QRET, FRET, FP and luminescence quenching in conventional bioaffinity and novel array formats.
Our current major interest is in protein aggregation and degradation studies using the Protein-Probe method. The technique is 100-fold more sensitive than Sypro Orange and can detect < 0.1% of antibody aggregate among intact antibody solution.
Protein-Probe
Label-free methods for protein stability, protein-ligand and protein-protein interaction studies have increased dramatically over the years. We have answered to the demand by developing luminescent label-free Protein-Probe assays using time-resolved luminescence detection and specific lanthanide probes. We found 100-fold improved sensitivity compared to existing technologies such as ThermoFluor using Sypro Orange as an external dye. This allows affordable protein studies and reduces risk for measuring aggregation at high protein concentration.
Peptide-Block
We have recently developed a single-peptide method for the detection of protein posttranslational modifications (PTM). Previously, by breaking the two-peptide complex enzymatically with added or removed PTM required structural integrity of leucine-zipper complex limiting the recognition sequence incorporation to the substrate peptide. The Peptide-FRET method relies on a single-peptide approach without any structural limitation while FRET measurement provides the basis of the detection.
Peptide-Break
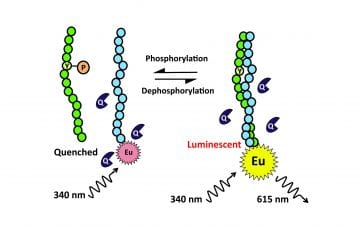
Enzymatic protein posttranslational modifications (PTM) such as phosphorylation, acetylation, glycosylation and ubiquitination, are vital cellular control mechanisms. Each modification is typically measured with a specific separate luminescent method today. We have developed a homogeneous high throughput screening method as a global method for the detection of potential all PTMs. The developed method is based on modifications in the leucine-zipper peptide-peptide binding properties and subsequent luminescence signal change. The main advantage of the developed method is the wide applicability to measure multiple different PTMs and targets. We have utilized i.a. the following LZ peptide structures in the study:
Eu-chelate labeled KDTLQAETDQLEDEKSALQTEIANLLKEKEKLEFIL
Substrate sequence REELRKRRAELRRRSAQLRQRREQLRRRSANLRKE Serine phosphorylation
QRET detection
Luminometric methods have been the primary detection technologies in the high throughput screening of novel drug candidates. Optical reading is simple, inexpensive and fast and is applicable to the screening of hundreds of thousands or even millions of small molecules. We have developed a homogeneous Quenching Resonance Energy Transfer (QRET) technology, which relies on the luminescence quenching of non-bound fraction of the labeled ligand while the bound fraction generates detectable luminescence signal. The technology has been applied to drug screening of multiple targets and its main advantage is the single label approach without the labeling of the target protein.
Chemical arrays
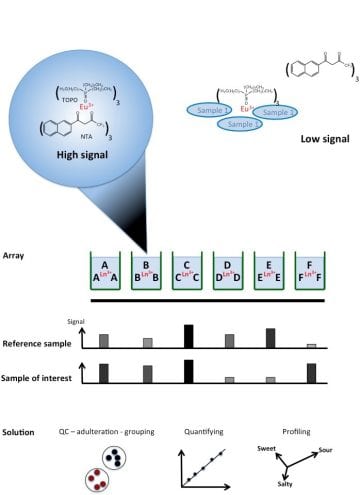
Nonspecific methods are capable of detecting holistic view over a sample and changes in the sample content. Our approach is homogeneous and based on the interaction of an array of chosen lanthanide chelates with the sample components. The resulting luminescent fingerprint is evaluated with multiparametric analysis and compared to the known reference samples to analyze e.g. changes in the sample content and clarifying authenticity or adulteration of i.a. honey adulteration, distinguishing cocoa species, follow-up of natural waters and detection of lung cancer etc.
Nanoparticle sensors
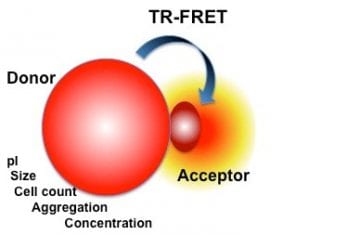
We have developed a homogeneous detection technique based on the nonspecific adsorption of analyte to nanoparticles and luminescence resonance energy transfer (LRET) detection principle to the quantification of chemical and biochemical molecules.
Our method relies on the adsorption competition of sample analyte molecule and labeled protein onto the surface of the nanoparticle. Upon binding onto the nanoparticle a LRET signal modulation occurs. The developed method is by far more sensitive than the most sensitive commercial methods, approx. 100-fold.