Research
Current research in physical chemistry focuses on bioinspired systems, deep eutectic solvents, functional layer-by-layer films, and special techniques in spectroelectrochemistry and NMR. The research in physical chemistry is phenomenon-oriented, it strives to understand the reasons for the observed properties and behavior, to create a model of the system. However, applications will not be forgotten, and the goals of the work are in biodegradable electronics, sustainable solvent systems, and in some bioapplications. If you are interested in working in this environment at any level of your studies, please contact prof. Jukka Lukkari.
The following list shows active and recent research areas in physical chemistry. More detailed description of each theme is found in the individual pages.
Bioinspired compounds and materials
We focus on quinonoid compounds, which are of fundamental importance for the energy metabolism in living cells. 1,4- and 1,2-benzoquinone (p– and o-benzoquinones, respectively) are the basic molecules in this field.
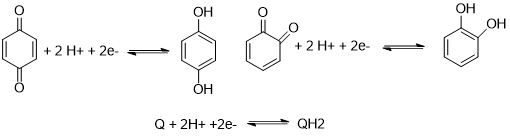
In spite of the apparent simplicity, their redox processes are, in fact, quite complicated and sensitive to many external stimuli. Our work concentrates on melanin-type materials, especially their synthetic counterpart, polydopamine (polyDOPA). We have worked out a detailed kinetic and thermodynamic model of the oxidation of dopamine (DA) by oxygen or redox-active transition metal ions, and characterized the properties of the resulting material.
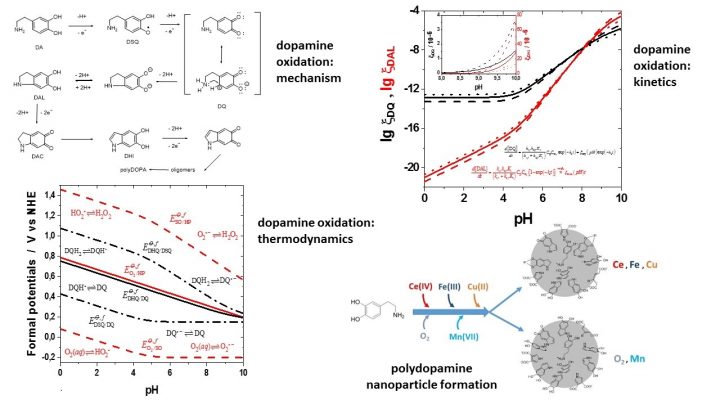
Current work aims at better understanding the redox processes of polydopamine, in the preparation and study of polymeric quinones, studying the preparation of their thin films using layer-by-layer techniques, and in the use of these materials in biodegradable electronics.
Relevant publications:
Mikko Salomäki, Lauri Marttila, Henri Kivelä, Tuomo Ouvinen, Jukka Lukkari*; Effects of pH and Oxidants on the First Steps of Polydopamine Formation: A Thermodynamic Approach, J. Phys. Chem. B 2018, 122(24), 6314-6327; doi: 10.1021/acs.jpcb.8b02304
Mikko Salomäki, Tuomo Ouvinen , Lauri Marttila, Henri Kivelä, Jarkko Leiro, Ermei Mäkilä, Jukka Lukkari*; Polydopamine Nanoparticles Prepared Using Redox-Active Transition Metals, J. Phys. Chem. B 2019, 123(11), 2513-2524; doi: 10.1021/acs.jpcb.8b11994
Biodegradable electronics
The electronic waste (e-waste) is a growing problem and its diverse and complex nature hinders attempts to resolve the problem. One promising and feasible partial solution to this problem is the emerging field of transient electronics. It refers to devices intended for short-term use, which decompose afterwards as a result of external conditions (e.g., moisture). The most important area in this field is biodegradable electronics. Biodegradable materials and components are degraded by micro-organisms or living body and produce only non-toxic and harmless products. This approach is also called sustainable or green electronics, and it is applicable, e.g., to temporary implantable biomedical devices, swarms of widely distributable environmental sensors, and transient devices in consumer packages.
An essential component of most electronic devices is the power source. Batteries and supercapacitors are appropriate power supplies for transient purposes, which do not require high energy and power output. Relatively little work has been done on biodegradable power sources, their materials, fabrication and performance enhancement but quinonoid compounds, especially eumelanin and its synthetic analogue polydopamine, have attracted attention as cost-effective bioinspired high energy density materials. Our research focuses on biodegradable supercapacitors, their materials, fabrication, and the fundamental science of redox processes in them.
Our current work has utilized oxidative multilayers as general platforms for capacitive films but further work will focus on other approaches, too. PEDOT-based films have been used as simple model systems in our studies and the results have been promising but films based on polydopamine have shown even higher capacitance values.
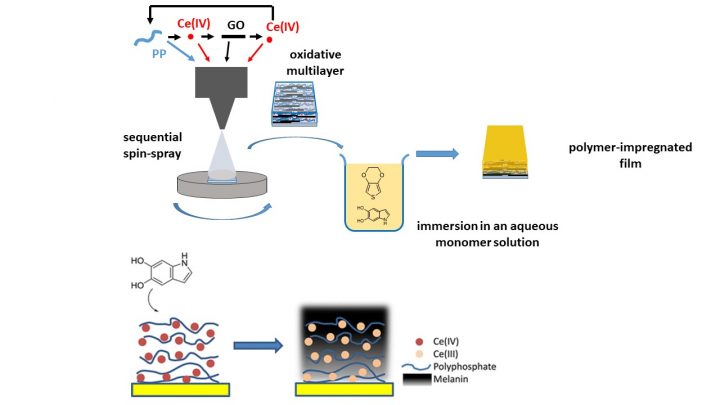
Relevant publications:
Mikko Salomäki, Lauri Marttila, Henri Kivelä, Matti Tupala, Jukka Lukkari; Oxidative Spin-Spray-Assembled Coordinative Multilayers as Platforms for Capacitive Films, Langmuir 2020, 36(24), 6736-6748; doi: 10.1021/acs.langmuir.0c00824
Deep eutectic solvents (DESs)
Deep Eutectic Solvents are liquid solvents, which are biocompatible, nontoxic, easily prepared from cheap reagents, have low vapour pressure and wide application range, i.e., ideal solvent systems for sustainable chemistry. Our work focuses on two-component hydrophilic and hydrophobic DESs of type III and, especially, their use in electrochemistry and the role of small water content on their properties. The work is a collaboration with the University of Modena and Reggio Emilia in Modena, Italy.
When working in the ambient atmosphere small amounts of water are always absorbed in the solvent. However, the role of water in small concentrations (below ca. 0.1 mole fraction) has, so far, hardly been discussed in literature. The work in progress deals with the fundamental role of water on the macroscopic and microscopic properties and structure of hydrophilic and hydrophobic DESs. In this work, we use conventional physicochemical techniques, NMR spectroscopy, and dynamic molecular simulations.
DESs are interesting solvents for electrochemical applications. Our work deals with their use as ion conductive media in biodegradable electronics and in environmental analysis. Hydrophobic DESs are suitable for extracting harmful species from waste water but their high viscosity hampers their use in electrochemical detection. In spite of this, we have, for the first time, used these solvents in electrochemical measurements and detection of extracted chromium species.
Relevant publications:
S. Ruggeri, F. Poletti, C. Zanardi, L. Pigani, B. Zanfrognini, E. Corsi, N. Dossi, M. Salomäki, H. Kivelä, J. Lukkari, F. Terzi; Chemical and electrochemical properties of a hydrophobic deep eutectic solvent, Electrochim. Acta, 2019, 295, 124-129; doi: 10.1016/j.electacta.2018.10.086
4 other manuscripts at various stages of preparation.
Layer-by-layer thin films
Layer-by-layer techniques for the fabrication of thin multilayers
Sequential layer-by-layer (LbL) assembly is a very versatile and easy method to produce thin films of a wide range of materials. In its simplest form, it is carried out by dipping the substrate repeatedly in solutions of oppositely charged components (dip-LbL). However, spraying the components on a spinning substrate (spi-spray-LbL) significantly speeds up the process and produces smoother and more stratified films.
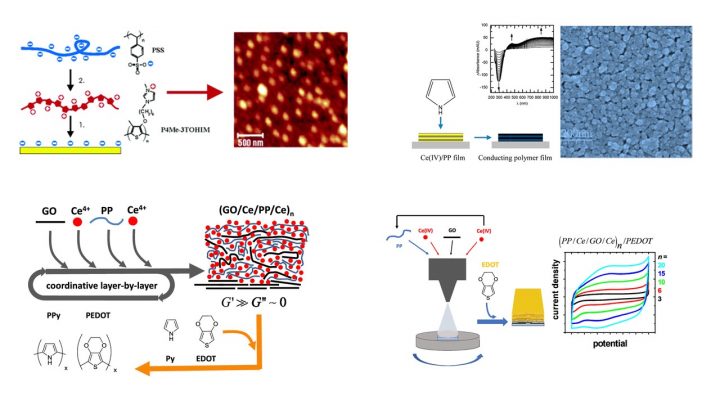
We have studied LbL techniques and films for ca. 20 years. The early work focused on the preparation and properties of films containing electronically conducting components, e.g., water-soluble conducting polythiophenes and single-wall carbon nanotubes. Our present research interest is in oxidative multilayers, which are rare examples of chemically reactive LbL films. Oxidative multilayers provide a general platform for a site-selective electrodeless generation of conducting polymer or melanin-type films on solid substrates. Our research has studied the effect of the LbL method, multilayer composition, thickness and other relevant factors on the kinetics, thermodynamics of the polymer film formation, and on its mechanical, optical and electronic properties.
Relevant publications:
Jukka Lukkari, Mikko Salomäki, Antti Viinikanoja, Timo Ääritalo, Janika Paukkunen, Natalia Kocharova, Jouko Kankare; Polyelectrolyte Multilayers Prepared from Water-Soluble Poly(alkoxythiophene) Derivatives, J. Am. Chem. Soc. 2001, 123(25), 6083-6091; DOI: 10.1021/ja0043486
Jukka Lukkari, Mikko Salomäki, Timo Ääritalo, Kari Loikas, Taina Laiho, Jouko Kankare; Preparation of Multilayers Containing Conjugated Thiophene-Based Polyelectrolytes. Layer-by-Layer Assembly and Viscoelastic Properties, Langmuir 2002, 18(22), 8496-8502; DOI: 10.1021/la025916t
Antti Viinikanoja, Jukka Lukkari, Timo Ääritalo, Taina Laiho, Jouko Kankare; Phosphonic Acid Derivatized Polythiophene: A Building Block for Metal Phosphonate and Polyelectrolyte Multilayers, Langmuir 2003, 19(7), 2768-2775; DOI: 10.1021/la0266738
Antti Viinikanoja, Sami Areva, Natalia Kocharova, Timo Ääritalo, Maarit Vuorinen, Arto Savunen, Jouko Kankare, Jukka Lukkari; Structure of Self-Assembled Multilayers Prepared from Water-Soluble Polythiophenes, Langmuir 2006, 22(14), 6078-6086; DOI: 10.1021/la060519u
Hanna Paloniemi, Marjo Lukkarinen, Timo Ääritalo, Sami Areva, Jarkko Leiro, Markku Heinonen, Keijo Haapakka, Jukka Lukkari; Layer-by-Layer Electrostatic Self-Assembly of Single-Wall Carbon Nanotube Polyelectrolytes, Langmuir 2006, 22(1), 74-83; DOI: 10.1021/la051736i
Fabio Terzi, Chiara Zanardi, Barbara Zanfrognini, Laura Pigani, Renato Seeber, Jukka Lukkari, Timo Ääritalo, Jouko Kankare; Preparation and Characterization of a Redox Multilayer Film Containing Au Nanoparticles, J. Phys. Chem. C 2009, 113(12), 4868-4874; DOI: 10.1021/jp809402j
Zanardi, Chiara; Terzi, Fabio; Zanfrognini, Barbara; Pigani, Laura; Seeber, Renato; Lukkari, Jukka; Ääritalo, Timo; Effective catalytic electrode system based on polyviologen and Au nanoparticles multilayer, Sens. Act. B-Chem. 2010, 144(1), 92-98; DOI: 10.1016/j.snb.2009.10.041
Zanfrognini, Barbara; Zanardi, Chiara; Terzi, Fabio; Ääritalo, Timo; Viinikanoja, Antti; Lukkari, Jukka; Seeber, Renato; Layer-by-layer deposition of a polythiophene/Au nanoparticles multilayer with effective electrochemical properties, J. Solid State Electrochem. 2011, 15(11-12), 2395-2400; DOI: 10.1007/s10008-011-1479-4
Mikko Salomäki, Markus Räsänen, Jarkko Leiro, Terhi Huti, Mikko Tenho, Jukka Lukkari, Jouko Kankare; Oxidative Inorganic Multilayers for Polypyrrole Film Generation, Adv. Func. Mat. 2010, 20(13), 2140-2147; DOI: 10.1002/adfm.200902412
Mikko Salomäki, Ossi Myllymäki, Minna Hätönen, Juho Savolainen, Jukka Lukkari; Layer-by-Layer Assembled Oxidative Films as General Platform for Electrodeless Formation of Conducting Polymers, ACS Appl. Mater. Interfaces 2014, 6(4), 2325-2334; DOI: 10.1021/am404342b
Mikko Salomäki, Matti Tupala, Timo Parviainen, Jarkko Leiro, Maarit Karonen, Jukka Lukkari; Preparation of Thin Melanin-Type Films by Surface-Controlled Oxidation, Langmuir 2016, 32(16), 4103-4112; DOI: 10.1021/acs.langmuir.6b00402
Mikko Salomäki, Jussi Kauppila, Jouko Kankare, Jukka Lukkari; Oxidative Layer-By-Layer Multilayers Based on Metal Coordination: Influence of Intervening Graphene Oxide Layers, Langmuir 2018, 34(44), 13171-13182; DOI: 10.1021/acs.langmuir.8b02784
Spectroelectrochemistry
Special spectroelectrochemical techniques
Modulated electroreflectance spectroscopy is a rarely used technique, in which the potential of the working electrode is sinusoidally modulated and the resulting modulation in the intensity of the reflected light is measured. The measurement can be done in ac-spectral, ac-reflectometry or reflectoimpedance modes. It is an extremely sensitive technique for studying redox-active layers, even below monomolecular coverage, and can be used to measure spectra of surface species, charge transfer kinetics, and redox processes selectively and without interference from capacitive effects.
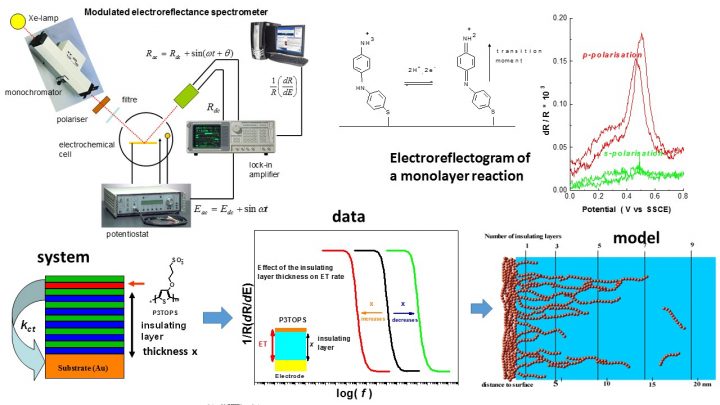
Photocurrent spectroscopy is a more familiar technique suitable for films mainly on semiconductor electrodes (although metal electrodes can be used in certain cases, too). It is also an extremely sensitive technique, allowing photocurrent measurements down to picoamp range.
Relevant publications:
Jukka Lukkari, Mikko Salomäki, Antti Viinikanoja, Timo Ääritalo, Janika Paukkunen, Natalia Kocharova, Jouko Kankare; Polyelectrolyte Multilayers Prepared from Water-Soluble Poly(alkoxythiophene) Derivatives, J. Am. Chem. Soc. 2001, 123(25), 6083-6091; DOI: 10.1021/ja0043486
Rapta, P; Lukkari, J; Tarabek, J; Salomaki, M; Jussila, M; Yohannes, G; Riekkola, ML; Kankare, J; Dunsch, L; Ultrathin polyelectrolyte multilayers: in situ ESR/UV-Vis-NIR spectroelectrochemical study of charge carriers formed under oxidation, PhysChemChemPhys 2004, 6(2), 434-441; DOI: 10.1039/b308891j
Jukka Lukkari, Mika Alanko, Virpi Pitkänen, Kari Kleemola, Jouko Kankare; Photocurrent Spectroscopic Study of the Initiation and Growth of Poly(3-methylthiophene) Films on Electrode Surfaces with Different Adsorption Properties, J. Phys. Chem. 1994, 98(34), 8525-8535; DOI: 10.1021/j100085a036
Jukka Lukkari, Jouko Kankare; Photocurrent spectroscopy of conducting polymers, Synth. Met. 1995, 69(1-3), 353-354; DOI: 10.1016/0379-6779(94)02482-E
Applied NMR
Applied NMR Spectroscopy at the Physical Chemistry group
Introduction
NMR spectroscopy, or nuclear magnetic resonance spectroscopy, is a spectroscopic research technique which detects the presence of magnetic atomic nuclei in the studied sample, by placing the sample in a strong external magnetic field and letting the nuclei of interest absorb and re-emit radio-frequency (RF) radiation (Fig. 1).
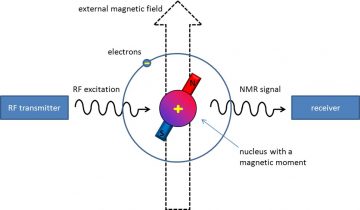
Figure 1. The nuclear magnetic resonance (NMR) phenomenon.
For a nucleus to be magnetic it needs to be “spinning”, i.e. its spin quantum number I has to be non-zero. The (nuclear ground state-) value of I depends only on the proton and neutron numbers Z and N of the nucleus, meaning that I is a nuclide-specific property much like mass or electric charge. Most chemical elements have at least one isotope with a magnetic and, therefore, NMR-active nucleus. For example, the most abundant hydrogen isotope 1H (with 99.98% natural abundance) has an NMR-active nucleus, proton (I = ½), and this gives rise to 1H NMR spectroscopy.
The RF signal emitted by the nuclear ensemble of interest during an NMR experiment is called the NMR signal or FID signal (free induction decay), and it contains information about the sample. On modern FT NMR instruments, this signal is acquired as a function of time (Fig. 2).
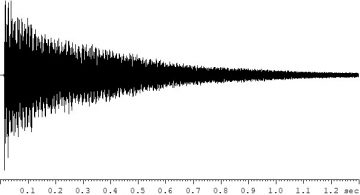
Figure 2. A typical time-domain 1H NMR signal (FID signal). The signal decays (roughly exponentially) due to nuclear relaxation processes following the RF excitation pulse.
The frequencies present in the detected NMR signal can be analyzed by a technique called Fourier Transform (FT) which results in a frequency-domain NMR spectrum (Fig. 3).
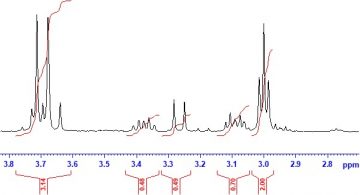
Figure 3. A typical 1H NMR spectrum (here, the frequencies are expressed in ppm relative to the 1H resonance frequency of the internal reference compound tetramethylsilane and the peak integrals are given in red).
The NMR spectrum displays a peak at a certain frequency value if this frequency component was present in the original time-domain NMR signal. The frequency of the peak depends on the type of nucleus and its chemical environment (→ structural information), while the intensity or integral of the peak is proportional to the concentration of the nucleus in the sample (→ quantitative information). Line widths and -shapes can also contain useful information, e.g. about the molecular motion and kinetics taking place within the sample. Thus, the frequencies, intensities and line widths of the peaks in the NMR spectrum, and their dependence on the experimental variables (such as temperature, time, concentration, and pH) can provide a great deal of information about the chemical structure, thermodynamics, kinetics, dynamics, reactions and interactions within the sample under study. NMR signal intensities can also be made to depend on the RF pulse-sequence parameters and magnetic field gradients in a variety of ways, which enables an extremely diverse and powerful set of NMR experiments and applications, e.g. 2D/multidimensional spectroscopy, T1/T2 relaxation time measurements, diffusion measurements, and magnetic resonance imaging (MRI).
Facilities
The new joint UTU and ÅA chemistry department building, Aurum, houses five modern Bruker FT-NMR instruments (400 MHz, 3×500 MHz, and 600 MHz) providing excellent facilities for NMR spectroscopic research. With this instrumentation, it is possible to measure all the commonly occurring NMR-active nuclear species and conduct most modern 1D, 2D (and nD) NMR experiments, including those using field gradients, using either solution or solid-state samples.
Applications
NMR spectroscopy can be applied to the determination of various properties of organic, inorganic and organometallic molecules, polymers, nanomaterials and mixtures in (gas,) liquid, and solid phases. The information is extracted from the NMR spectral parameters such as
the chemical shift, J-coupling constant, nuclear Overhauser effect (NOE), signal intensity and line width, and T1 and T2 relaxation times, as well as from their dependence on experimental parameters such as time, temperature, field-gradient strength and other factors.
The topics and materials studied with NMR spectroscopy at the Physical Chemistry group either are due to the group itself, or relate to collaboration with local research groups at departments of chemistry and physics, or external research institutions. These applications include:
The assessment and determination of molecular structure, including 3D structure elucidation of molecules in solution (NMR spectroscopy is uniquely suited for solution-state 3D structure determination!). Examples of our research includes structure determination of substituted fullerenes (Fig. 4), and metal-organic coordination compounds (in collaboration with Inorganic Materials Chemistry group).
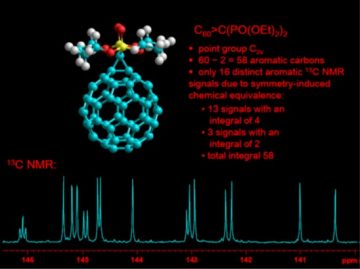
Figure 4. Symmetry-guided 13C NMR spectroscopic structure determination of a substituted C60 fullerene. Note also the triplet splitting in the downfield peak (consisting of four chemically equivalent carbons), due to J-coupling to two chemically equivalent 31P nuclei.
Size determination of macromolecules. For example, identification of polymer (such as polyphosphate) end-group NMR signals and the comparison of their integrals with the signals from the repeat units allows for the estimation of average chain length.
Chemical kinetics and thermodynamics. Quantitative NMR measurements allow for the determination of relative equilibrium concentrations in an equilibrium mixture (and identification of the species present at equilibrium!), as a function of temperature as needed, so that equilibrium constants and the related thermodynamic parameters can be determined. Quantitative NMR measurements as a function of time (and temperature) for non-equilibrium systems enable the study of kinetics. Fast kinetics may be studied with line shape analysis.
Solid-state NMR spectroscopy. E.g. the physical state and gradual s/l phase transition of drugs such as ibuprofen confined in mesoporous silicon have been observed and analyzed with the help of suitably-parameterized 13C ss-NMR experiments (Fig. 5) (in collaboration with Industrial Physics). Confinement in mesopores can improve the dissolution and uptake properties of (slightly-soluble) drugs.
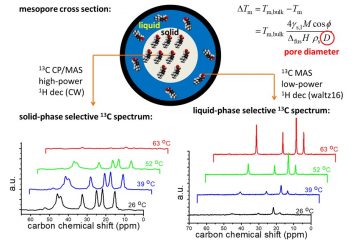
Figure 5. Gradual phase transition of ibuprofen confined in mesoporous (ca. 10+ nm in diameter) silicon as seen by suitably modified 13C ss-NMR spectroscopy.
Diffusion NMR spectroscopy. The diffusion properties (self-diffusion coefficients, hydrodynamic radii, …) e.g. of polymers, nanoparticles, and deep eutectic solvent (DES) components have been studied by using diffusion NMR spectroscopy (Fig. 6). Furthermore, this technique allows for the separation of the individual spectra of mixture components by use of DOSY processing (diffusion-ordered NMR spectroscopy).
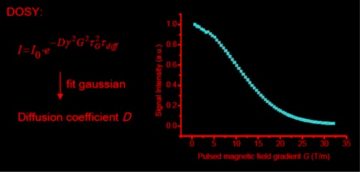
Figure 6. The characteristic Gaussian (bell curve) dependence of NMR spectral peak integral on the field gradient strength used in the diffusion NMR measurement allows for the determination of D.
T1/T2 relaxation time measurements. Information about local molecular dynamics and rotational correlation times can be extracted from the transverse (T2) and longitudinal (T1) relaxation times of nuclear magnetization (and their temperature dependence). This we have applied e.g. in the study of deep eutectic solvent (DES) mixtures.
Personnel
Henri Kivelä, university teacher, Ph.D.
Conducting polymers
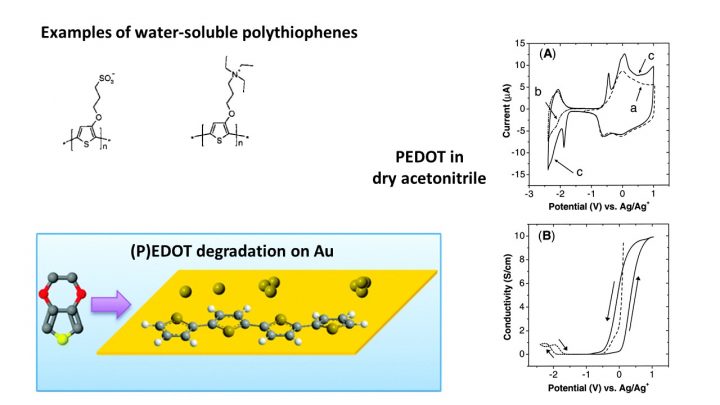
Conducting polymers are everyday materials in research but not anymore a research item themselves. We have studied the fundamental redox proeprties of polythiophenes, including PEDOT, and polypyrrole, and studied their interaction with metal surfaces. The latter has important implications for their use together with metal nanoparticles in electrocatalytic and –analytic applications.
Relevant publications:
Harri J. Ahonen, Jukka Lukkari, Jouko Kankare; n- and p-Doped Poly(3,4-ethylenedioxythiophene): Two Electronically Conducting States of the Polymer, Macromolecules 2000, 33(18), 6787-6793; DOI: 10.1021/ma0004312
Fabio Terzi, Luca Pasquali, Monica Montecchi, Stefano Nannarone, Antti Viinikanoja, Timo Ääritalo, Mikko Salomäki, Jukka Lukkari, Bryan P. Doyle, Renato Seeber; New Insights on the Interaction between Thiophene Derivatives and Au Surfaces. The Case of 3,4-Ethylenedioxythiophene and the Relevant Polymer, J. Phys. Chem. C 2011, 115(36), 17836-17844; DOI: 10.1021/jp203219b
Jukka Lukkari, Mikko Salomäki, Antti Viinikanoja, Timo Ääritalo, Janika Paukkunen, Natalia Kocharova, Jouko Kankare; Polyelectrolyte Multilayers Prepared from Water-Soluble Poly(alkoxythiophene) Derivatives, J. Am. Chem. Soc. 2001, 123(25), 6083-6091; DOI: 10.1021/ja0043486
Rapta, P; Lukkari, J; Tarabek, J; Salomaki, M; Jussila, M; Yohannes, G; Riekkola, ML; Kankare, J; Dunsch, L; Ultrathin polyelectrolyte multilayers: in situ ESR/UV-Vis-NIR spectroelectrochemical study of charge carriers formed under oxidation, PhysChemChemPhys 2004, 6(2), 434-441; DOI: 10.1039/b308891j
Jukka Lukkari, Mika Alanko, Virpi Pitkänen, Kari Kleemola, Jouko Kankare; Photocurrent Spectroscopic Study of the Initiation and Growth of Poly(3-methylthiophene) Films on Electrode Surfaces with Different Adsorption Properties, J. Phys. Chem. 1994, 98(34), 8525-8535; DOI: 10.1021/j100085a036
Carboneous materials
Carbon materials (carbon nanotubes, graphene materials)
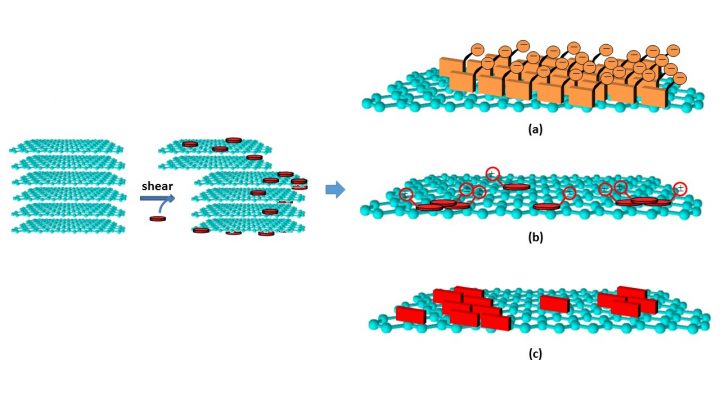
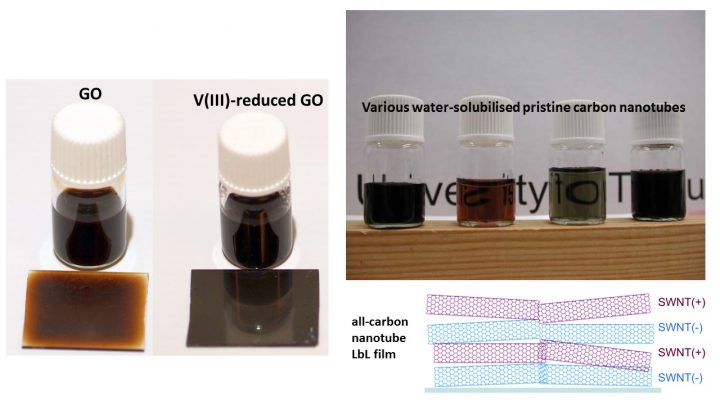
These materials are not actively studied any more but they belong to the toolbox of current research. We have used them in layer-by-layer film and chemical sensors, studied the solubilization of single-walled carbon nanotubes in aqueous solutions using non-covalent modification, the direct exfoliation of graphite to graphene and the chemical reduction of graphene oxide.
Relevant publications:
Viinikanoja, Antti; Kauppila, Jussi; Damlin, Pia; Mäkilä, Ermei; Leiro, Jarkko; Ääritalo, Timo; Lukkari, Jukka; Interactions between graphene sheets and ionic molecules used for the shear-assisted exfoliation of natural graphite, Carbon 2014, 68, 195-209; DOI: 10.1016/j.carbon.2013.10.080
Kauppila, Jussi; Lund, Liisa; Laiho, Taina; Salomäki, Mikko; Kankare, Jouko; Lukkari, Jukka; Effective low temperature reduction of graphene oxide with vanadium(III), J. Mater. Chem. C 2014, 2(18), 3602-3609; DOI: 10.1039/c4tc00142g
Hassinen, Jukka; Kauppila, Jussi; Leiro, Jarkko; Määttänen, Anni; Ihalainen, Petri; Peltonen, Jouko; Lukkari, Jukka; Low-cost reduced graphene oxide-based conductometric nitrogen dioxide-sensitive sensor on paper, Anal. Bioanal. Chem. 2013, 405(11), 3611-3617; DOI: 10.1007/s00216-013-6805-5
Self-assembled monolayers
Self-assembled monolayers (SAMs)
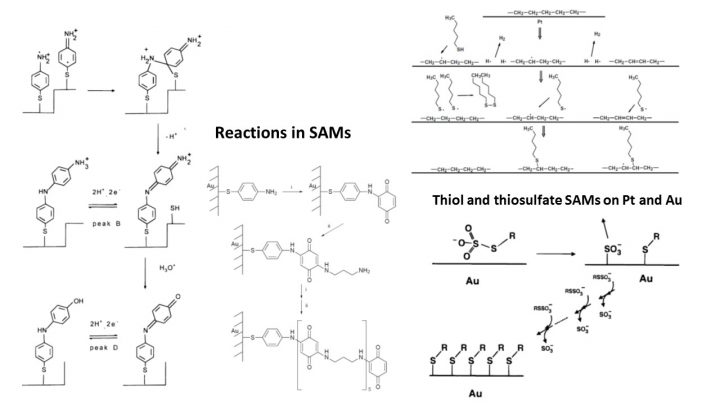
SAMs are not studied any more themselves but they are used throughout the current research. Our work has focused on the stability of SAMs and their chemical reactions.
Relevant publications:
Jukka Lukkari, Kari Kleemola, Minna Meretoja, Tapio Ollonqvist, Jouko Kankare; Electrochemical Post-Self-Assembly Transformation of 4-Aminothiophenol Monolayers on Gold Electrodes, Langmuir 1998, 14(7), 1705-1715; DOI: 10.1021/la970931x
Jukka Lukkari, Minna Meretoja, Ilkka Kartio, Kari Laajalehto, Markku Rajamäki, Mia Lindström, Jouko Kankare; Organic Thiosulfates (Bunte Salts): Novel Surface-Active Sulfur Compounds for the Preparation of Self-Assembled Monolayers on Gold, Langmuir 1999, 15(10), 3529-3537; DOI: 10.1021/la9811719
Laiho, T; Lukkari, J; Meretoja, M; Laajalehto, K; Kankare, J; Leiro, JA; Chemisorption of alkyl thiols and S-alkyl thiosulfates on Pt(111) and polycrystalline platinum surfaces, Surf. Sci. 2005, 584(1), 83-89; DOI: 10.1016/j.susc.2005.02.059
Laiho, T; Leiro, JA; Lukkari, J; XPS study of irradiation damage and different metal-sulfur bonds in dodecanethiol monolayers on gold and platinum surfaces, Appl. Surf. Sci. 2003, 212, 525-529; DOI: 10.1016/S0169-4332(03)00462-8
Lukkari, J; Kleemola, K; Meretoja, M; Kankare, J; Polyaminoquinone self-assembled films on electrode: Synthesis of all-organic molecular wires by solution phase epitaxy, Chem. Commun. 1997, 1099-1100; DOI: 10.1039/a701895i
Lanthanoid luminescence
Luminescent lanthanoid chelates
Lanthanoid luminescence was an active field of research but it is ending with the final Ph.D. Thesis in 2020. The research has been focused on the energy transfer pathways and the bioapplications of the luminescent chelates.
Relevant publications:
Räsänen, M., Takalo, H., Rosenberg, J., Mäkelä, J., Haapakka, K., Kankare, J.; Study on photophysical properties of Eu(III) complexes with aromatic beta-diketones Role of charge transfer states in the energy migration, J. Lumin. 2014, 146, 211-217; DOI 10.1016/j.jlumin.2013.09.076
Räsänen, M., Takalo, H., Rosenberg, J., Soukka, T., Haapakka, K., Kankare, J.; Photophysical study of blue-light excitable ternary Eu(III) complexes and their encapsulation into polystyrene nanoparticles, J. Lumin. 2015, 160, 128-133; DOI 10.1016/j.jlumin.2014.12.001
Räsänen, M., Rosenberg, J., Lukkari, J., Haapakka, K., Kankare, J., Takalo, H.; Study on luminescent ternary EuEDTA complexes with a set of substituted 4-phenylethynyl and 4-aryl pyridine-2,6-dicarboxylic acids, J. Lumin. 2017, 187, 471-478; DOI 10.1016/j.jlumin.2017.03.061
Räsänen, M., Takalo, H., Rosenberg, J., Haapakka, K., Lukkari, J., Kankare, J.; Energy transfer in ternary TbEDTA chelates with a series of dipicolinic acid derivatives, J. Lumin. 2020, 220, 116967; DOI 10.1016/j.jlumin.2019.116967